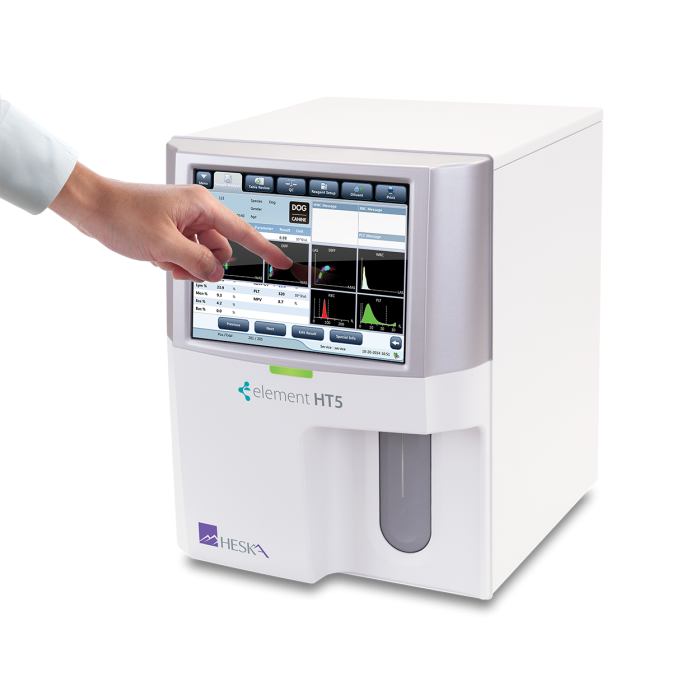
Element HT5®
Veterinary Hematology Analyzer
A true 5-part CBC differential in under 1 minute.
-
Touchscreen Interface
Large color touch screen with intuitive navigation, simplifies patient data entry and results viewing.
-
Easy Reagent Management
Simple reagent management via bar-code scanner and on-board menu functions, with internal space saving reagent storage.
-
Rapid Results
Results for 20 parameters in under 60 seconds.
-
A Trifecta of Proven Technology
Using flow cytometry, impedance, and colorimetry, the Element HT5 provides fast and accurate results including a true 5-part WBC differential.
-
Multiple Species Availability
Dog, Cat, Horse, Cow, Ferret, Goat, Llama, Camel, Monkey, Mouse, Pig, Rabbit, Rat, Sheep, Giant Panda, and Red Panda.
-
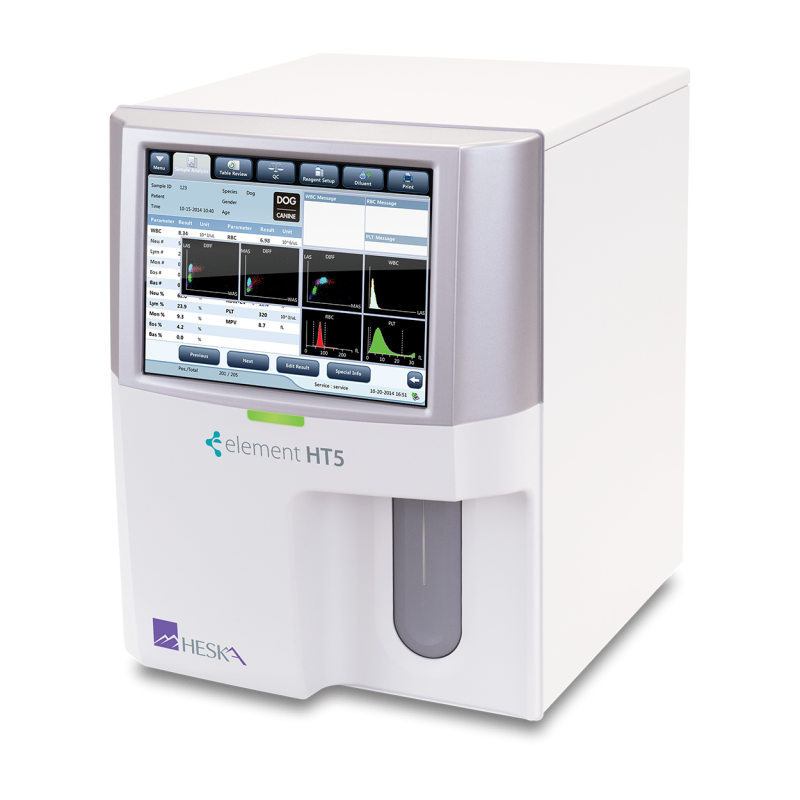
Intuitive Navigation
Simplified data entry and test review with numeric results, histograms and scatter plots.
True 5-Part: Laser + Impedance + Colorimetric Hematology
Proven Accuracy and Reproducibility
Excellent correlation for a population of animals measured on two different technologies.
Multiple Species Options
Dog, Cat, Horse, Cow, Ferret, Goat, Llama, Camel, Monkey, Mouse, Pig, Rabbit, Rat, Sheep, Giant and Red Panda.
Triple Angle Laser Scatter
Detects complexity and granularity of cell volumes.
Simple Data Management
Assign, unassign or delete multiple items at once with easy web-style navigation links.
Sample Pathology Messages
Intelligent information messages related to pathology present in the sample.
Excellent Correlation
Achieved for a population of animals measured on two different technologies.
Technical Details & Downloads
Available Species:
Dog, Cat, Horse, Cow, Ferret, Goat, Llama, Camel, Monkey, Mouse, Pig, Rabbit, Rat, Sheep, Giant Panda, and Red Panda.
Interfaces:
4 USB ports
1 Ethernet Port
Power Supply:
Voltage Input power Frequency: Analyzer (100V-240V~)±10% 300 VA (50Hz/60Hz)±1Hz
Fuse:
WARNING: Use specified fuse only. Fuse specification: 250V T3.15AH
EMC Description:
Do not use this device in close proximity to sources of strong electromagnetic radiation (e.g., unshielded intentional RF sources), as these may interfere with the proper operation. This equipment complies with the emission and immunity requirements of the EN61326–1:2006 and EN61326–2–6:2006.
NOTE: It is the manufacturer’s responsibility to provide equipment electromagnetic compatibility information to the customer or user.
NOTE: It is the user’s responsibility to ensure that a compatible electromagnetic environment for the equipment can be maintained in order that the device will perform as intended.
Sound:
Maximal sound: 65 dBA
Operating Environment:
Optimal operating temperature: 50°F – 86°F
Optimal operating humidity: 20% – 85%
Atmospheric pressure: 70 kPa – 106 kPa
Storage Environment:
Ambient temperature: 15°F – 104°F
Relative humidity: 10% – 90%
Atmospheric pressure: 50 kPa – 106 kPa
Running Environment:
Ambient temperature: 50°F – 104°F
Relative humidity: 10% – 90%
Atmospheric pressure: 70 kPa – 106 kPa
NOTE: Be sure to use and store the analyzer in the specified environment
Best-in-Class Technology
3 Measurement Methods Used:
- Electrical Impedance method for determining the RBC and PLT data
- Colorimetric Method for determining the HGB
- Flow Cytometry by Laser for determining the WBC data
- Other parameter results are obtained via calculation

1. Electrical Impedance Method
RBCs/PLTs are counted and sized by the Electrical Impedance method. This method is based on the measurement of changes in electrical resistance produced by a particle, which in this case is a blood cell, suspended in a conductive diluent as it passes through an aperture of known dimensions. A pair of electrodes is submerged in the liquid on both sides of the aperture to create an electrical pathway. As each particle passes through the aperture, a transitory change in the resistance between the electrodes is produced. This change produces a measurable electrical pulse. The number of pulses generated represents the number of particles that passed through the aperture. The amplitude of each pulse is proportional to the volume of each particle.
Output includes numeric values, percentages plus histograms for RBC, WBC & PLT plus scatter plots for analyzing cell volume, complexity and granularity.
2. Flow Cytometry by Laser
After a predetermined volume of blood is aspirated and diluted by a certain amount of reagent, it is injected into the flow cell. Surrounded with sheath fluid (diluent), the blood cells pass through the center of the flow cell in a single column at a faster speed. When the blood cells suspended in the diluent pass through the flow cell, they are exposed to a laser beam.
The intensity of scatter light reflects the blood cell size and intracellular density. The low-angle scattered light reflects cell size, and the high-angle scattered light reflects intracellular density (nucleus size and density). The optical detector receives this scatter light and converts it into electrical pulses. Pulse data collected can be used to draw a 3-dimensional distribution (scattergram).
Triple angle scatter detects cell volume, complexity and granularity of cells.
- Low Angle Scatter (LAS)-Detects Cell Volume
- Mid Angle Scatter (MAS)- Detects Cellular Complexity
- Wide Angle Scatter (WAS)-Detects Cellular Granularity
- Multiple angle scatterplots aid to differentiate WBC differential
3. Colorimetric Method
The WBC/HGB dilution is delivered to the HGB bath where it is bubble mixed with a certain amount of lyse, which converts hemoglobin to a hemoglobin complex that is measurable at 530 nm. An LED is mounted on one side of the bath and emits a beam of monochromatic light, whose central wavelength is 530 nm. The light passes through the sample and is then measured by an optical sensor that is mounted on the opposite side. The signal is then amplified and the voltage is measured and compared to the blank reference reading (readings taken when there is only diluent in the bath), and the HGB is measured and calculated in the analyzer automatically.
Reticulocytes
Anemia is typically present in 5–10% of small animal samples. In these instances, it is useful to evaluate the blood for the presence of regeneration and RBC abnormalities that may help determine the cause of the anemia. Heska recommends that the best way to assess regeneration is examination of the stained blood film for polychromasia (or reticulocytes), while also looking for RBC abnormalities. The Element HT5 analyzer’s identification of an increase in RDW and/or MCV is evidence of probable reticulocytosis, but this is best confirmed by slide examination.
Sample Pathology Messages
The Element HT5 system provides intelligent information messages related to pathology present in the sample. These messages are intended to be a guide to sample result criteria for slide review that may be used in a laboratory. These messages are indications that additional diagnostic information may be found on the slide to supplement the instrument results.
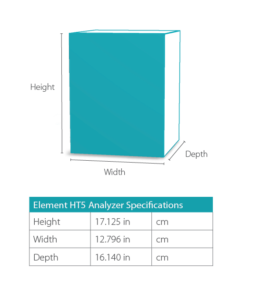
Dimensions
Instructions for Control File Download:
- Right click on the control file.
- Click on Save Link As…or Save Target As…depending on your browser.
Save to USB drive and then upload to Analyzer.
Control Lot BC2501B, exp 03/10/2025
Normal-Control
Assay Sheet PDF
Tri-Level-Controls
Control Lot BC2411B, exp 01/10/2025
Normal-Control
Assay Sheet PDF
Tri-Level-Controls
Heska Support Teams are Here for You
We’re Available When You Need Us
Rest assured that when you need help, have questions, or have difficulties, we have you covered.
- Available 6:00am – 5:00pm MST Daily
- Emergency Support During Off-Hours
- Case Consultations or Product Usage Help